Insights,
Insights,
Part B Discarded Drug Units for 2021
The Centers for Medicare and Medicaid Services (CMS) requires all providers and suppliers to report the “JW” HCPCS modifier on Part B drug claims for discarded drugs and biologicals beginning January 1, 2017. CMS reports aggregate discarded units, also referred to as “wastage,” through the Medicare Part B Discarded Drug Units Dashboard. Beginning in 2023, as required by the Infrastructure Investment and Jobs Act,1 manufacturers must provide a refund to the federal government for discarded units that are in excess of 10% of the total allowed amount for Part B drugs.
CMS is required to implement this policy through notice and comment rulemaking2 and included implementation and operationalization guidance for the discarded unit policy in the CY 2023 Medicare Physician Fee Schedule (MPFS) proposed rule.3 To help manufacturers and stakeholders prepare, ADVI SAVEs analyzed CY 2021 Medicare FFS Part B drug claims to determine which drugs may be at risk for owing a rebate on discarded units.
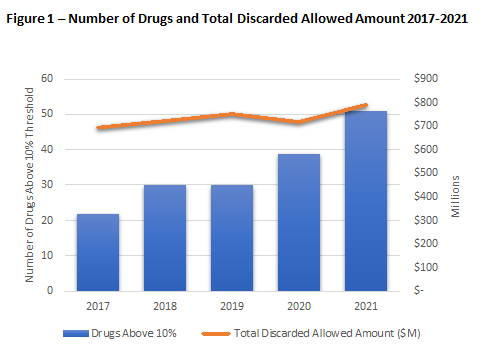
In the 2020 Part B Discarded Drug Units Dashboard, CMS identified 39 drugs that have a discarded unit’s percentage above 10%. This is nearly double the number of drugs above the threshold since CMS started tracking discarded units in 2017 (see Figure 1).4 In 2020, an additional 15 drugs had a discarded unit’s percentage between 8.0-10.0% nearing the penalty threshold.
In replicating CMS’ methodology for claims in 20215, ADVI SAVEs found 51 drugs with a discarded units percentage greater than 10% (listed in Table 1 below) and 10 additional drugs with a discarded percentage between 8-10% (Table 2 below). There are thirteen new drugs with discarded units above 10% in 2021; six of them were not on the market in 2020. Additionally, three drugs were above the 10% threshold in 2020 have now dropped below in 20216.
Table 1 – Part B Drugs with Discarded Units Above 10% Threshold7
|
HCPCS |
Brand Name |
Generic Name |
2021 Total Allowed Amount |
2021 Total Allowed Amount for Units Discarded |
2021 Discarded Units as % of Total Allowed Amount |
2020 CMS Discarded Units as % of Total Allowed Amount |
Newly Above Discarded Unit Threshold in 2021 |
| Q9965 | Omnipaque | Iohexol |
$2,288,078 |
$1,547,573 |
67.6% |
23.4% |
|
| J7342 | Otiprio | Ciprofloxacin |
$4,560 |
$2,429 |
53.3% |
n/a8 |
Yes |
| J9281 | Jelmyto | Mitomycin |
$26,599,582 |
$10,006,372 |
37.6% |
n/a6 |
Yes |
| J9262 | Synribo | Omacetaxine Mepesuccinate |
$220,987 |
$68,454 |
31.0% |
20.0% |
|
| J9043 | Jevtana | Cabazitaxel |
$146,571,165 |
$43,694,280 |
29.8% |
28.1% |
|
| J9041 | Velcade | Bortezomib |
$379,992,118 |
$104,493,105 |
27.5% |
26.7% |
|
| J9351 | Hycamtin | Topotecan HCL |
$474,014 |
$124,918 |
26.4% |
23.4% |
|
| Q9961 | Conray | Iothalamate Meglumine |
$19,263 |
$4,979 |
25.8% |
18.0% |
|
| J0894 | Dacogen | Decitabine |
$17,861,716 |
$4,383,499 |
24.5% |
22.7% |
|
| J9044 | Bortezomib | Bortezomib |
$4,614,083 |
$1,045,731 |
22.7% |
21.8% |
|
| J9025 | Azacitidine | Azacitidine |
$37,953,273 |
$8,464,431 |
22.3% |
22.0% |
|
| J9017 | Arsenic Trioxide | Arsenic Trioxide |
$1,734,221 |
$371,806 |
21.4% |
18.3% |
|
| J0775 | Xiaflex | Collagenase Clostridium Hist. |
$68,213,415 |
$14,289,396 |
20.9% |
20.2% |
|
| J1448 | Cosela | Trilaciclib |
$1,731,620 |
$361,554 |
20.9% |
n/a6 |
Yes |
| J9065 | Cladribine | Cladribine |
$448,801 |
$92,569 |
20.6% |
18.1% |
|
| J9223 | Zepzelca | Lurbinectedin |
$90,554,293 |
$18,650,080 |
20.6% |
n/a6 |
Yes |
| J0565 | Zinplava | Bezlotoxumab |
$3,902,449 |
$774,415 |
19.8% |
19.6% |
|
| J9178 | Ellence | Epirubicin HCL |
$9,923 |
$1,880 |
18.9% |
12.2% |
|
| J9229 | Besponsa | Inotuzumab Ozogamicin |
$17,827,073 |
$3,245,314 |
18.2% |
12.1% |
|
| Q4121 | Theraskin, per sq cm | n/a9 |
$6,534,621 |
$1,153,492 |
17.7% |
7.2% |
Yes |
| J0223 | Givlaari | Givosiran Sodium |
$10,700,177 |
$1,859,208 |
17.4% |
20.8% |
|
| Q9966 | Isovue-200 | Iopamidol |
$2,224,432 |
$375,791 |
16.9% |
30.1% |
|
| Q4106 | Dermagraft | Cult Skin Subst,human-Bovine |
$1,500,661 |
$251,210 |
16.7% |
15.1% |
|
| J1640 | Panhematin | Hemin |
$8,351,097 |
$1,311,570 |
15.7% |
14.9% |
|
| J9153 | Vyxeos | Daunorubicin/Cytarabine Lipos |
$5,519,948 |
$862,395 |
15.6% |
14.6% |
|
| J2425 | Kepivance | Palifermin |
$124,548 |
$19,075 |
15.3% |
8.4% |
Yes |
| Q4101 | Apligraf | Cult Skin Subst, human-Bovine |
$2,167,077 |
$318,419 |
14.7% |
12.1% |
|
| J9264 | Abraxane | Paclitaxel Protein-Bound |
$347,082,269 |
$49,289,715 |
14.2% |
14.5% |
|
| J0122 | Xerava | Eravacycline Di-Hydrochloride |
$110,760 |
$15,667 |
14.1% |
1.0% |
Yes |
| J9027 | Clofarabine | Clofarabine |
$62,487 |
$8,749 |
14.0% |
1.3% |
Yes |
| J2796 | NPlate | Romiplostim |
$257,086,834 |
$35,660,702 |
13.9% |
16.8% |
|
| J0515 | Benztropine Mesylate | Benztropine Mesylate |
$16,479 |
$2,178 |
13.2% |
8.2% |
Yes |
| Q9956 | OptIson | Perflutren Protein-A Microsphr |
$735,086 |
$96,158 |
13.1% |
11.8% |
|
| J2562 | Mozobil | Plerixafor |
$18,708,893 |
$2,437,455 |
13.0% |
12.4% |
|
| J9179 | Halaven | Eribulin Mesylate |
$43,558,916 |
$5,670,164 |
13.0% |
12.6% |
|
| J9307 | Folotyn | Pralatrexate |
$22,832,179 |
$2,903,611 |
12.7% |
10.3% |
|
| J9037 | Blenrep | Belantamab |
$32,989,426 |
$4,156,354 |
12.6% |
n/a6 |
Yes |
| J9269 | Elzonris | Tagraxofusp-Erzs |
$7,753,098 |
$952,097 |
12.3% |
6.8% |
Yes |
| J0485 | Nulojix | Belatacept |
$76,726,554 |
$9,403,607 |
12.3% |
11.4% |
|
| J9042 | Adcetris | Brentuximab Vedotin |
$169,124,286 |
$20,461,098 |
12.1% |
11.4% |
|
| J9319 | Istodax | Romidepsin |
$6,512,543 |
$777,333 |
11.9% |
n/a6 |
Yes |
| J9205 | Onivyde | Irinotecan Liposomal |
$59,364,736 |
$7,027,567 |
11.8% |
10.5% |
|
| J3396 | Visudyne | Verteporfin |
$2,557,468 |
$302,023 |
11.8% |
9.5% |
Yes |
| Q9950 | Lumason | Sulfur Hexafluoride Microsphr |
$512,606 |
$60,471 |
11.8% |
13.0% |
|
| J9228 | Yervoy | Ipilimumab |
$417,585,675 |
$48,488,372 |
11.6% |
10.1% |
|
| J2997 | Activase | Alteplase |
$66,073,440 |
$7,547,395 |
11.4% |
11.3% |
|
| J3241 | Tepezza | Teprotumumab-Trbw |
$306,354,597 |
$34,853,650 |
11.4% |
10.0% |
|
| J3300 | Triesence | Triamcinolone Acetonide/PF |
$8,955,625 |
$983,566 |
11.0% |
11.4% |
|
| J3101 | Tnkase | Tenecteplase |
$12,849,516 |
$1,374,852 |
10.7% |
6.2% |
|
| J9315 | Istodax | Romidepsin |
$23,128,462 |
$2,403,694 |
10.4% |
10.3% |
|
| J9352 | Yondelis | Trabectedin |
$9,222,158 |
$933,528 |
10.1% |
11.0% |
Table 2 – Part B Drugs with Discarded Units Nearing 10% Threshold (>8%)10
|
HCPCS |
Brand Name |
Generic Name |
2021 Total Allowed Amount |
2021 Total Allowed Amount for Units Discarded |
2021 Discarded Units as % of Total Allowed Amount |
2020 CMS Discarded Units as % of Total Allowed Amount |
| J0594 | Busulfan | Busulfan |
$65,247 |
$6,506 |
10.0% |
6.6% |
| J9203 | Mylotarg | Gemtuzumab Ozogamicin |
$2,970,017 |
$294,047 |
9.9% |
5.5% |
| J9263 | Oxaliplatin | Oxaliplatin |
$3,995,999 |
$387,153 |
9.7% |
9.4% |
| J0641 | Fusilev | Levoleucovorin Calcium |
$454,127 |
$41,406 |
9.1% |
9.0% |
| J9355 | Herceptin | Trastuzumab |
$232,099,376 |
$20,425,837 |
8.8% |
8.6% |
| J0585 | Botox | Onabotulinumtoxina |
$369,462,938 |
$32,181,718 |
8.7% |
8.3% |
| J9400 | Zaltrap | Ziv-Aflibercept |
$1,593,900 |
$137,978 |
8.7% |
9.8% |
| J2786 | Cinqair | Reslizumab |
$11,899,277 |
$981,743 |
8.3% |
8.5% |
| J0222 | Onpattro | Patisiran Sodium,lipid Complex |
$83,468,489 |
$6,869,746 |
8.2% |
8.4% |
| J9309 | Polivy | Polatuzumab Vedotin-Piiq |
$43,939,303 |
$3,570,913 |
8.1% |
15.8% |
Future Research
ADVI is following CMS guidance on implementation of the Part B drug discarded units policy. With our access to near real-time Medicare claims data, we are able to analyze specific drugs dosing patterns in both the outpatient hospital and physician office setting to better understand the discarded unit’s issue. We can aggregate results at the patient, physician, group practice, or hospital outpatient provider level and have claims from CY2017 through June of 2022.
If you are interested in additional analysis related to Part B drug discarded units, please contact Caitlin Sheetz (Caitlin.sheetz@advi.com), Pete Kardel (Peter.kardel@advi.com), or your ADVI account manager.
1 P.L. 117-58, §90004: https://www.congress.gov/117/plaws/publ58/PLAW-117publ58.pdf
2 SSA §1847A(h)(7)
3 CY 2023 Payment Policies under the Physician Fee Schedule and Other Changes to Part B Payment Policies: https://public-inspection.federalregister.gov/2022-14562.pdf
4 Not all 39 drugs would meet the proposed definition of refundable single-dose container or single-use package drug.
5 ADVI used the Research Identifiable Files (RIFs) from January through December 2021. These are not 100% final actions claims and due to varying run out times, results may be slightly different than future published dashboards.
6 These drugs are: Q4195 (Puraply, per square centimeter), J9309 (Polivy [Polatuzumab Vedotin-Piiq]), and J0291 (Zemdri [Plazomicin Sulfate). J9309 has a 2021 discarded units of over 8.1% and appears in Table 2 as a drug nearing the discarded unit threshold.
7 Table 1 presents claims billed with the JW HCPCS modifier and does not account for any of the discarded unit policy exceptions under P.L. 117-58. Reach out to ADVI for more information about whether specific products would be affected by the discarded unit rebate policy.
8 Drug not sold in 2020.
9 Q4121 – Brand/generic name not applicable. Name reflects the HCPCS short descriptor.
10 Table 2 presents claims billed with the JW HCPCS modifier and does not account for any of the discarded unit policy exceptions under P.L. 117-58. Reach out to ADVI for more information about whether specific products would be affected by the discarded unit rebate policy.
$251,210


Head of Policy, Research, and Analysis