Insights,
Insights,
On November 2, 2023, the Centers for Medicare and Medicaid Services (CMS) issued the 2024 Medicare Hospital Outpatient Prospective Payment System (OPPS) final rule (link) with an accompanying fact sheet (link).
Final Rule highlights include:
(Note: With respect to the Physician Fee Schedule Final Rule and the ADVI Instant you received last night, CMS has confirmed to the AMA that the 2024 Medicare conversion factor is $32.7442, not $32.7375 as identified in Table 116 and as previously reported. The decrease from the 2023 conversion factor is 3.37%.)
340B
CY 2024 Payment Policy
For CY 2024, CMS is finalizing its proposal to continue to pay the statutory default rate of ASP+6% for 340B acquired drugs and biologicals (consistent with CMS policy for drugs and biologicals not acquired through the 340B program).
CMS refers stakeholders to the “Hospital Outpatient Prospective Payment System Remedy for the 340B-Acquired Drug Payment Policy for CY 2018-2022” final rule (link). While the final rule does not include changes to the CY 2024 OPPS drug payment policy or conversion factor, CMS notes that it does include changes to the calculation of the conversion factor beginning in CY 2026. See our ADVI Instant on the final remedy rule for more information.
Use of “JG” and “TB” Modifiers
In the CY 2023 OPPS final rule, CMS maintained the requirement that 340B hospitals report the “JG” or “TB” modifiers to identify drugs acquired through the 340B Program for informational purposes. In the December 2022 “Part B Inflation Rebate Guidance: Use of 340B Modifiers” guidance, CMS upheld this policy, noting that it provided an existing mechanism to identify units of drugs and biologicals acquired through 340B so they can be subtracted from Part B inflationary rebates under the Inflation Reduction Act (IRA).
However, CMS is finalizing its proposal to instead use a single modifier, with the stated aim of simplifying the process for providers. Effective January 1, 2025, all 340B covered entity hospitals paid under OPPS will be required to report the “TB” modifier, even if the hospital previously reported the “JG” modifier. CMS states that the “JG” modifier will remain in use through December 31, 2024, but hospitals may choose to transition to the “TB” modifier anytime in 2024. CMS notes that they plan to update the “Part B Inflation Rebate Guidance: Use of 340B Modifiers” guidance with this change and to align modifier requirements for 340B entity providers and suppliers not paid under the OPPS.
Hospital Transparency
CMS finalized its proposal to update the requirements for hospitals to make public their standard charges and CMS enforcement of hospital price transparency regulations. Finalized proposals include a requirement for hospitals to display their standard charge information by conforming to a CMS template layout, data specifications, and data dictionary. Enforcement process changes include requiring submission of certification by an authorized hospital official as to the accuracy and completeness of the data, requiring the acknowledgment of receipt of a warning notice, and publicly posting information related to hospital compliance. CMS also finalized a phased enforcement approach. All finalized changes will be effective January 1, 2024; however, the regulation text specifies later dates by which hospitals must comply with some of these new requirements. CMS will begin enforcing hospital compliance with those new requirements on the applicable compliance dates.
Device Pass-Through Status
For CY 2024, CMS received six device pass-through payment applications by March 1, 2023 (the last quarterly deadline for applications to be included in the CY 2024 OPPS proposed rule). CMS approved four of the applications and denied two.
Drug Packaging Threshold
CMS finalized its proposal with modification, keeping the drug packaging threshold at $135 per day for CY 2024. This is a $5 decrease from the proposal to increase the threshold to $140 per day. Items with a per day cost greater than $135 are identified as separately payable unless they are policy-packaged.
Biosimilars
Drugs and Biologicals with Expiring Pass-Through Payment Status in 2024
CMS is finalizing its proposal to end pass-through payment status in CY 2024 for 25 drugs and biologicals. The products were initially approved for pass-through payment status between April 1, 2021, and January 1, 2022, and are listed in Table 90 of the unpublished final rule.
CMS finalized its proposal to continue pass-through payment status in CY 2024 for 42 drugs and biologicals. These products were approved for pass-through payment status between April 1, 2022, and October 1, 2023, and are listed in Table 91 of the unpublished final rule.
Enhancing Oncology Model (EOM)
CMS sought comments on proposed APC and status indicator assignments for new HCPCS codes established and made effective on April 1, 2023. This includes “M0010: Enhancing oncology model (EOM) monthly enhanced oncology services (MEOS) payment for EOM enhanced services”. The full list of status indicators and definitions can be found in Addendum D1 of the final rule.
Changes to the List of ASC Covered Surgical Procedures
CMS added 37 surgical procedures to the ASC CY 2024 covered procedures list (CPL). Included are 26 dental procedures and 11 additional procedures such as total shoulder arthroplasty (TSA) [HCPCS 23472]. CMS finalized its proposal to not remove any procedures from the ASC CPL in CY 2024.
Changes to the Inpatient Only (IPO) List
CMS finalized its proposal to not remove any services from the IPO list in CY 2024.
CMS finalized the proposal to assign CPT codes 0790T, 22836, 22837, 22838, 61889, 76984, 76987, 76988, 76989, and 0646T to status indicator “C” for CY 2024.

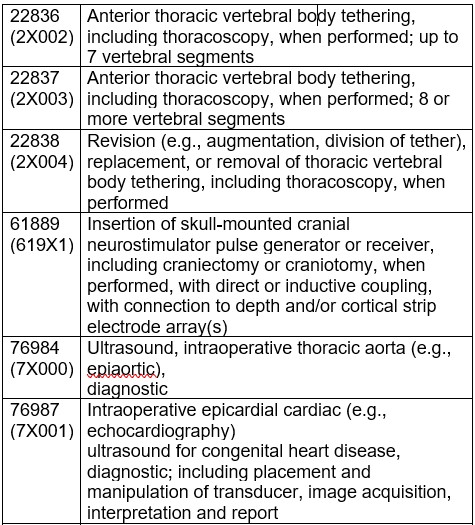
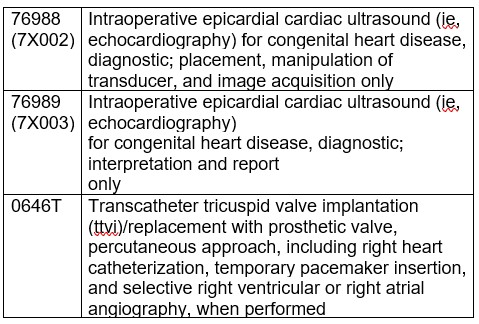
In the proposed rule, CMS solicited comments on whether the following 4 services are appropriate to be removed from the IPO list:
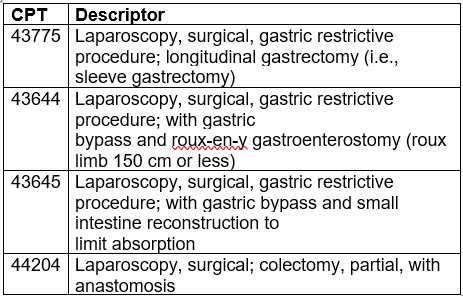
CMS notes they continue to believe these services do not meet the criteria to be removed from the IPO list and are therefore maintaining CPT codes 43775, 43644, 43645, and 44204 on the IPO list for CY 2024.
Partial Hospitalization Program and Intensive Outpatient Services
For CY 2024, CMS finalized its proposal to establish separate Ambulatory Payment Classification (APC) per diem payment rates for Partial Hospitalization Program (PHP) days with 3 services and 4 or more services and to establish separate APC per diem payment rates for Community Mental Health Centers (CMHCs) and hospital-based PHPs. CMS will make payments at the 3-service rate for PHP or intensive outpatient services (IOP) days that have fewer than 3 services. CMS is also finalizing a requirement that at least one service must be from the PHP and IOP Services list to qualify for payment for the PHP or IOP APC; these services are listed in Table 99 of the unpublished final rule.
CMS finalized its proposal to require the physician certification for PHP services include a certification that the patient requires such services for a minimum of 20 hours per week.
In the unpublished final rule, CMS establishes payment and program requirements for the IOP benefit. CMS describes patient eligibility for IOP as patients who have a mental health diagnosis, are likely to benefit from coordinated services, do not require 24-hour care, are not a danger to themselves or others, have a support system while not in treatment, and have the cognitive and emotional ability to participate in treatment. CMS also clarifies that Medicare covers PHP for treatment of substance use disorders.
CMS notes that the following services are to be covered under the IOP: Individual and group therapy with physicians or psychologists; occupational therapy; services of social workers, psychiatric nurses, and other trained psychiatric staff; drugs and biologicals prescribed for therapeutic purposes; individualized activity therapy that are not recreational; family counseling; patient training and education; and diagnostic services. The following services are not paid under IOP: physician services, physician assistant services, nurse practitioner and clinical nurse specialist services, qualified psychologist services, and skilled nursing facility-furnished services. CMS finalized IOP regulations be covered and centrally incorporated into the remit for qualifying CMHCs (under Medicare Part B).
Payment for Non-Opioid Products Under Section 6082 of the SUPPORT Act
The HHS Secretary continues to evaluate payment terms of opioid and non-opioid treatments to remove any instances of potential financial incentivization toward opioid use. For CY 2024, CMS is finalizing a drug package threshold payment of $135 (a $5 decrease from the proposed rule) for treatments that are FDA-approved for pain management (unpackaged as surgical supplies with no transitional pass-through payment status), coverage for which was established by CY 2022 OPPS/ ASC final rule.
In the CY 2023 OPPS final rule, CMS finalized five drugs that would receive separate payment in the ASC setting for CY 2023: HCPCS codes C9290 (Exparel), J1096 (Dextenza), J1097 (Omidria), C9089 (Xaracoll), and C9144 (Posimir). In the CY 2024 OPPS proposed rule, CMS reevaluated these products and is finalizing its proposal that HCPCS codes C9290 (Exparel), J1096 (Dextenza), J1097 (Omidria), and C9089 (Xaracoll) continue to meet the required criteria and will receive separate payment in the ASC setting. CMS states that HCPCS code C9144 (Posimir) is payable through OPPS transitional pass-through status and will not be separately payable.
In the proposed rule, CMS requested comment for the CY 2025 OPPS rulemaking on the inclusion of and implementation for The Consolidated Appropriations Act, specifically the “Access to Non-Opioid Treatments for Pain Relief” section. CMS thanked commenters for their responses and noted that they would take suggestions into account for CY 2025 OPPS rulemaking.
Health Equity Consideration in OPPS Impacts
In the proposed rule, CMS sought feedback about the impact of OPPS and ASC changes to historically underserved populations (e.g., racially and socioeconomically diverse, federally recognized tribes, non-English speaking, people with disabilities, rural communities, LGBTQ+). Current OPPS impact data captured includes provider type, rural versus urban, geographic region, teaching status, and ownership type. CMS was interested in health equity questions that can be answered by those attributes, as well as soliciting ideas for further data points and categories to be collected. CMS welcomed suggestions around building and expanding a health equity framework and adding new health equity measures.
Commenters were supportive of incorporating health equity into future impact analyses. Suggestions include: identifying instances of negative payment policies; adding elements to address social drivers of health; using health equity accreditation programs or frameworks to assess payment adjustments or other health disparity impacts; researching beneficiary awareness of outpatient services; assessing utilization by geographic area and socioeconomic circumstances; outlining provider health equity goals, adopting certification requirements; recognizing essential hospitals in payment policies; considering hospital performance within a health equity framework and proportion of vulnerable populations served; and advancing interoperable data systems to collect health equity data.
Other Comment Solicitations
ADVI will continue monitoring developments and the next steps. This is a delayed release. ADVI Instant content is distributed in real-time for retainer clients. Get in touch to learn more about how we can support your commercialization, market access, and policy needs.


Head of Policy, Research, and Analysis