Insights,
Insights,
In recent years, states have increasingly enacted Prescription Drug Affordability Boards (PDABs). While their stated purpose is to increase affordability, there are concerns that PDABs could ultimately limit access to care, including for products treating rare diseases and rare conditions.
PDABs typically consist of around five members, appointed by the governor, with expertise in health policy, healthcare economics, or clinical medicine. Broadly, PDABs have two main functions: 1) to identify “high cost” drugs and 2) to recommend or implement policy solutions.
Some PDABs have the authority to set upper payment limits (UPLs), which would govern what purchasers can pay and state-regulated payers (e.g., individual, fully funded commercial, state employee plans, Medicaid) can reimburse for products dispensed or administered to patients in the state. Although self-funded plans regulated under the Employee Retirement Income Security Act of 1974 (ERISA) are generally excluded from UPL requirements, it’s likely many would choose to reimburse at the lower UPL rate. While states are technically not regulating manufacturers, the rationale behind UPLs is that manufacturers will lower their prices to continue selling products in the state.
PDABs with UPL authority go through four main processes: identification, selection, affordability review, and UPL determination.
Despite their smaller patient populations, products treating rare diseases or conditions may be disproportionately impacted by PDABs given that they may be more likely to meet eligibility criteria for selection. Additionally, patients with certain rare diseases or conditions are more likely to have commercial or Medicaid coverage. PDABs may select these products given their cost to the state, even if other non-rare products present a greater affordability challenge to consumers generally. Although products treating rare diseases or conditions often have limited therapeutic alternatives, only one state (Washington) currently exempts these treatments from UPLs. Other states require PDABs to “consider” a product’s orphan status in their review, but do not specify what this means.
While PDABs with the authority to set UPLs are likely to have the most impact, there are several other types with more limited authority. This includes:
2025 State of Play
Enacted PDABs
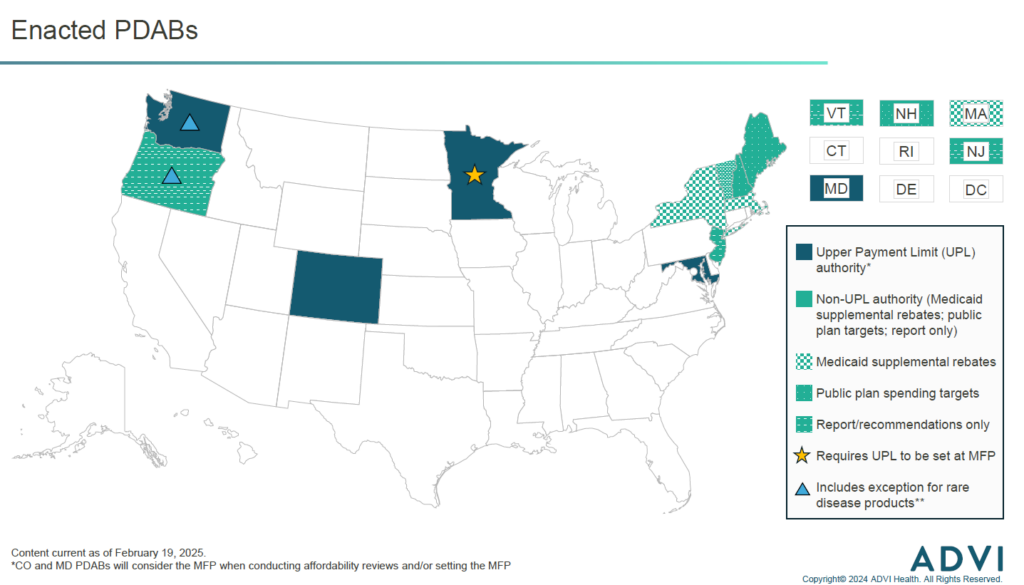
Colorado’s PDAB is the furthest along in the process of setting UPLs. The PDAB is moving forward with establishing UPLs for three of the five products it initially selected for affordability reviews. The board will hold rulemaking hearings throughout 2025 to establish the UPL amount for each product and determine how UPLs will be implemented. The five products the PDAB initially selected in 2023 included one rare disease product (Trikafta). However, after sizeable pushback from patients regarding the potential for reduced access, the board ultimately voted that Trikafta is “not unaffordable”, meaning it will not move forward with setting a UPL. Perhaps due in part to this pushback, in 2024 the Colorado legislature enacted legislation that requires the PDAB to consider input from consumers and the state’s Rare Disease Advisory Committee when considering drugs with orphan designation(s) for one or more rare diseases and no other indications.
Other PDABs with UPL authority, in Maryland and Washington, are moving forward with affordability reviews in 2025, and may choose to establish UPLs for the selected products. Minnesota’s PDAB was enacted in 2023 and is still focused on hiring staff and drafting policies for PDAB processes (e.g., identification, selection, affordability review).
While the Oregon PDAB does not have the authority to set UPLs, they will conduct affordability reviews of selected products in 2025. In late 2024, the PDAB submitted a statutorily mandated report to the state legislature outlining how UPLs could be established and implemented, if the OR legislature were to expand the PDAB’s authority. If the legislature were to do so, the PDAB’s prior experience in conducting affordability reviews could expedite the UPL process, especially compared to states establishing new PDABs.
Proposed PDABs
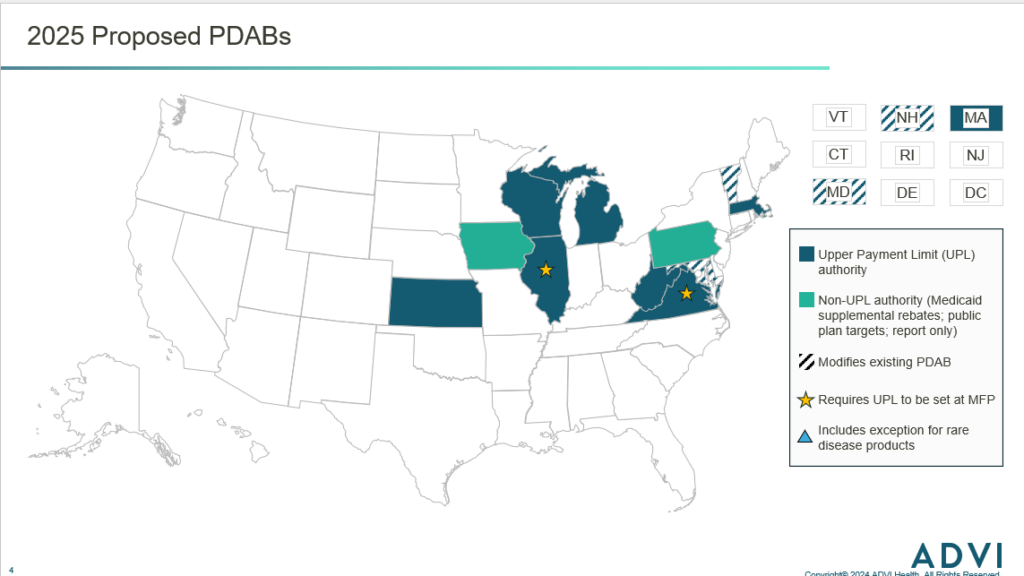
Several states have already proposed legislation to establish a PDAB in 2025. Most bills would create a PDAB with UPL authority, few would create PDABs that focus on public payers or only issue recommendations. Additionally, as of February 2025, no bills include exceptions for rare disease products, however more states are including provisions that would tie the UPL to the MFP under the IRA.
Outlook
In all, 2025 is expected to be an active year for PDABs, and activity will only increase in the future as more states pass legislation. While it is still uncertain how individual states will determine UPLs, products treating rare diseases or conditions may face additional challenges as more PDABs are enacted. Given limited exceptions, increased likelihood of meeting eligibility criteria, and potential for greater Medicaid spending, rare disease products may be disproportionately impacted by PDABs if states do not choose to exempt these products. If UPLs result in reduced access, patients with rare diseases will have fewer therapeutic alternatives to turn to. Additionally, if PDABs broadly target rare disease products, there may be a negative impact on innovation, particularly due to the increased investment required to develop these treatments.
The Federal and State Policy team will continue to follow this issue and key changes that may impact your business. Get in touch to gain expert insights and strategic counsel on the evolving healthcare landscape.


Director