ADVI Instant: CMS Releases Draft Part Two Guidance for the MPPP program
On February 15, the Centers for Medicare and Medicaid Services (CMS) released the Draft Part Two Guidance on the Medicare Prescription Payment Plan (MPPP) program (link). Both Parts One and Two guidance pertain to the first year of the program, contract year (CY) 2025.
In this draft part two guidance, CMS describes requirements for Part D sponsor obligations related to outreach and education, pharmacy processes, and operational considerations for the program.
CMS will accept comments on this draft guidance until March 16, 2024, and submitted to: PartDPaymentPolicy@cms.hhs.gov. CMS will issue the final part two guidance in the summer of 2024 after considering the public comments. CMS previously stated that it expects to issue the final part one guidance in Spring 2024 (see here for ADVI’s instant on the Draft Part One Guidance). Additionally, CMS will issue model materials specific to the program in summer 2024 following an Information Collection Request (ICR) through the Office of Management and Budget (OMB).
The Medicare Prescription Payment Plan implements the Inflation Reduction Act’s maximum monthly cap on cost-sharing payments under Prescription Drug Plans and MA-PD plans. The draft part one guidance detailed how Part D sponsors must allow elections, determine a maximum monthly cap, bill participants, and notify pharmacies when enrollees are likely to benefit from MPPP.
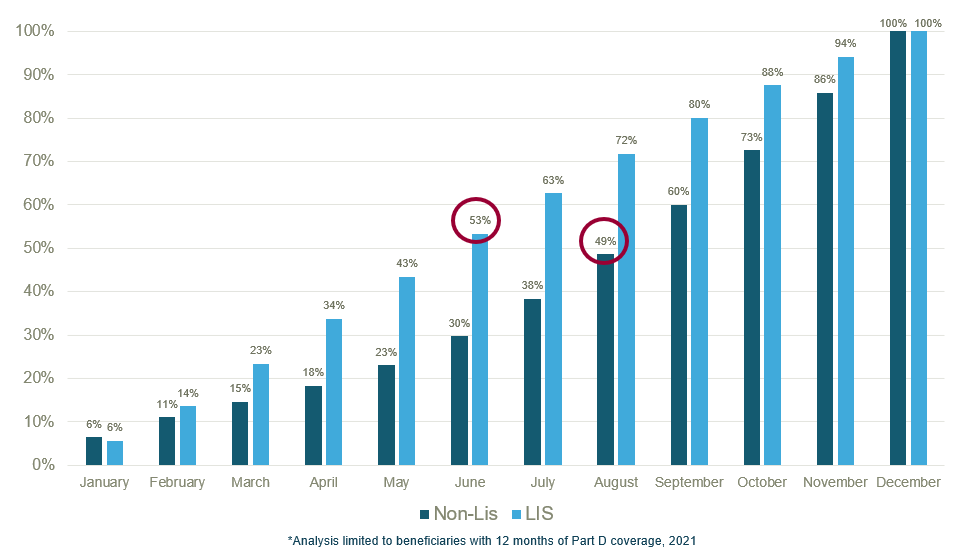
The draft part two guidance focuses on Part D sponsors obligations for MPPP education, outreach, and communication. The guidance notes that Part D enrollees who incur high out-of-pocket (OOP) costs earlier in the plan year are more likely to benefit from the MPPP. An analysis by ADVI SAVEs found that ~50% of LIS beneficiaries reach the $2,000 OOP threshold in June and ~50% of non-LIS beneficiaries reach the OOP threshold two months later in August.
When beneficiaries cross the threshold can vary substantially; to understand the cost-sharing journey for certain beneficiaries/beneficiaries on a certain product, reach out to ADVI SAVEs.
Outreach, Education, and Communications Requirements for Part D Sponsors
General Outreach and Education
- Part D sponsors must include information about the Medicare Prescription Payment Plan in the following communications:
- Mailing of membership ID cards (must include an MPPP election request form)
- The Evidence of Coverage (EOC)
- The Annual Notice of Change (ANOC)
- The Explanation of Benefits (EOB)
- Part D Sponsors’ website (must include an MPPP election mechanism)
- CMS is updating the model EOC, ANOC, and EOB materials, which will be released in spring 2024 as part of the general issuance of 2025 Model Materials.
- Part D sponsors are encouraged to use language from CMS-developed materials for all non-standardized communication materials (accompanying materials with the membership ID card and the Part D sponsor website), however, they are not required to do so.
Targeted Outreach and Education Requirements for Part D Sponsors
- Part D sponsors are required to have a mechanism to notify a pharmacy when a Part D enrollee incurs out-of-pocket (OOP) costs that make it likely the enrollee may benefit from participating in the MPPP.
- If a notification is received, Part D sponsors must then require the pharmacy to inform enrollees about the MPPP.
- CMS is requiring Part D sponsors to identify enrollees likely to benefit from the program and perform targeted outreach both before and during the plan year.
- In the year before the plan year, Part D sponsors must identify enrollees who incurred $2,000 in OOP costs for covered drugs through September of that year.
- During the plan year, Part D sponsors must target enrollees who are prescribed a new high-cost drug that would trigger the pharmacy point-of-service (POS) notification, if the Part D sponsor is made aware through prior authorization or utilization management edits in place for the drug.
- CMS is developing a standardized notice for “likely to benefit” enrollees that Part D sponsors will be required to use for outreach (via mail or electronically).
Communications with Program Participants and Model Materials Requirements
- MPPP Elections
- Part D sponsors must accept election requests they receive for MPPP, regardless of the format of the request (e.g., paper, telephone, website).
- Part D sponsors are encouraged to provide tailored examples for enrollees to understand their financial implications under MPPP.
- Part D sponsors are required to communicate that the request to participate in the MPPP has been accepted and effectuated (via written notice for requests in advance of the plan year, and telephonically then written notice for requests during the plan year).
- MPPP Notices
- CMS is developing model language for the following notices:
- Participation Request Form
- Notice of Acceptance of Election
- Notice for Failure to Make Payments under the MPPP
- Notification of Termination of Participation in the MPPP
- Notification of Voluntary Removal from the MPPP
- These model notices will be published for public comment and finalized by summer 2024 ahead of the CY 2025 Annual Election Period.
Language Access and Accessibility Requirements
- CMS requires outreach materials and communications be provided in a culturally competent manner to all Part D enrollees.
- Requirements stipulate Part D sponsors must provide translated materials to Part D enrollees on a standing basis in any non-English language that is the primary language of at least 5% of the individuals in a plan benefit package service area.
- Materials must also be provided in an accessible format using auxiliary aids and services upon request.
CMS Part D Enrollee Education and Outreach
- CMS states that it will update existing Part D educational resources with information on the MPPP, in addition to developing new materials.
- CMS notes that it will create an educational product intended for Part D enrollees on the Medicare website and other communication channels. Part D sponsors’ use of this educational product will satisfy the Part D sponsor requirement to provide information on the MPPP. CMS also encourages Part D sponsors to use the created product to provide information to pharmacies, contracted providers, and other interested parties, and to supplement their own educational materials.
- Supplemental Coverage
- In Part 1 of the MPPP guidance, CMS outlined a “likely to benefit” notification that will be required at the pharmacy point of service (POS) based on the OOP costs for a single prescription. The notification will be based on a determined OOP threshold, which will be finalized in the Final Part 1 guidance.
- In the Draft Part 2 guidance, CMS clarified that beneficiaries with supplemental coverage may not receive notifications about the MPPP because their OOP will be reduced below the threshold.
- CMS intends to include language in the “Medicare Prescription Payment Plan Likely to Benefit Notice” with recommendations for beneficiaries with supplemental insurance.
- Beneficiary Notification
- CMS recommends that Part D sponsors ensure their customer service representatives are aware of the following possibilities when communicating with beneficiaries:
- Beneficiaries with supplemental insurance may not receive notifications about the MPPP at POS.
- If a beneficiary enrolls in the MPPP late in a plan year, but has costs below the maximum monthly cap, they may be required to pay the full amount as part of their first month’s bill.
- In general, all MPPP requirements are the same across pharmacy types. However, in settings where there isn’t direct contract with enrollees the Part D sponsor must ensure that a hard copy of the “Medicare Prescription Payment Plan Likely to Benefit Notice” is provided to beneficiaries likely to benefit at the time the prescription is picked up.
- CMS outlines alternate strategies for pharmacies that do not have a practical method of providing a hard copy (i.e., long term care pharmacies, mail order pharmacies).
- CMS states that the requirement to notify certain beneficiaries of the MPPP should not be interpreted as a requirement to delay dispensing of medication.
- Readjudication of Claims for New Participants
- If an enrollee receives a “Medicare Prescription Payment Plan Likely to Benefit Notice” at pharmacy POS, they may choose to leave the pharmacy without their prescriptions and decide to enroll in MPPP at a later date.
- If a beneficiary opts to enroll in MPPP and returns to the pharmacy to pick up their prescriptions, CMS states that all claims for covered Part D drugs from prior dates of service that have not yet been paid for and picked up by the beneficiary must be readjudicated to allow for appropriate processing by the Part D sponsor and/or PBM.
- Processing Claims for Participants in Special Settings
- CMS outlines certain situations where a beneficiary may not benefit from the MPPP:
- Long term care pharmacies that currently bill the long-term care facility, not the beneficiary.
- Tribe and Tribal Organization, and Urban Indian Organization (I/T/U) Pharmacies that provide no-cost prescriptions to eligible enrollees.
Part D Sponsor Operational Requirements
- Part D Bidding Guidance for CY 2025
- Part D sponsors are required to report unsettled balances owed by participants under the MPPP as plan losses. If a Part D sponsor is compensated by a participant for the unsettled balance or sells the balance as debt, the amount is not considered a loss and must not be included in the bid.
- As a result, the Part D bid pricing toll will be modified by CMS to reflect projected losses for the MPPP. These losses must be reflected as administrative costs in the Part D BPT (link).
- Medical Loss Ratio (MLR) Instructions
- MA organizations and Part D sponsors are subject to fines and other penalties if they fail to have an MLR of at least 85%. MLR is calculated as a percentage of revenue used for patient care, not including administrative expenses or profit. MLR data is reported annually.
- Unsettled balances owed under MPPP are considered administrative costs and will not be included in MLR calculations.
- Monitoring and Compliance
- CMS will require Part D sponsors to report information related to MPPP through Part D Event (PDE) records and new reporting requirements.
- PDE guidance will be published in spring 2024
- Other reporting requirements are outlined in the Part 1 guidance
- CMS will monitor and collect data about beneficiary complaints through the Medicare Complaints Tracking Module (CTM) to assess compliance with MPPP requirements, beneficiary protections, and overall program integrity.
- Part D sponsors are expected to include MPPP in compliance programs to ensure that they are meeting program requirements.
- CMS may conduct audits of Part D sponsors’ implementation of MPPP and may request additional data collection or a site visit.
- Direct and Indirect Remuneration (DIR) Reporting Guidance
- Part D sponsors are required to report drug costs and DIR associated with the Part D benefit to CMS annually.
- No changes to DIR calculations or MPPP reporting are expected.
ADVI will continue monitoring developments and the next steps. This is a delayed release. ADVI Instant content is distributed in real-time for retainer clients. Get in touch to learn more about how we can support your commercialization, market access, and policy needs.